Remote Clinical Research Coordinator Jobs Hiring Now December 2022 Remote Part-Time, Full-Time, Online Jobs
Table of Content
Minimum of seven years relevant clinical research experience in... Collates materials for training and investigator meetings. Tracks site activation, enrolment and monitoring visits to projected plans, and escalate any issues or delays with site activation or deviations from monitoring plan. Clinical point of contact for scientific issues/questions for internal and external stakeholders (e.g., IRB, sites). BS/BA in Life Sciences with 7+ yrs clinical research experience. May lead or support a study or studies, depending on size/complexity.
Reporting to the Assistant Program Director, the Clinical Coordinator is responsible for ensuring that the service provision is of the highest quality and is consistent with housing first and harm reduction approach. Solomon Page has an extensive network of established clients which allows us to present opportunities that are well-suited to your respective goals and needs - this specialized approach sets us apart in the industries we serve. Founded in 1990, Solomon Page is a specialty niche provider of staffing and executive search solutions across a wide array of functions and industries.
VIETNAMESE-ENGLISH SPEAKING Clinical Assistant
The Clinical Coordinator oversees a staff of 4-6, liaises with psychiatric support services, interacts daily with multiple outreach providers, and serves as a member of the Program's management team. Develops and manages the budgeting, designing, planning, resource management, institutional review board, legal agreement with sites, purchasing, execution and management of clinical trials. Responsible for the execution and adaptation of clinical data management plans and processes for collection, management, reporting and analysis of data on behalf of project teams and sponsors. Clinical Research Director will provide leadership to ensure all operations support quality patient care and clinical research and comply with all Command, federal, state, regulatory, Defense & Veterans Brain Injury Center & contractor... Facilitate clinical evidence reviews with cross functional team to align on clinical evidence strategies, document / track decisions and risks, and write PMCF Plans. Support development and drafting of clinical study protocols, registry plans...
Read and understand clinical trial protocols to identify high-fidelity patient matches for various cancer types in both oncology and... The Clinical Research and Analytics role will have the critical responsibility of overseeing our efforts to measure and track the impact of Memora's care programs on delivering value to patients and care teams. We recommend you enter a location since many remote jobs have city, state or country requirements. Supports and adheres to laboratory standard operating procedures, to ensure Good Clinical Laboratory Practices , including careful documentation and strict protocol adherence. The coordinator is responsible for collecting data, such as the start and end date, how severe it is, how the medicine will be taken, and if there are any adverse events.
List of Jobs
Virtual clinical trials are going to stay in the field, and the technical and self knowledge you gain working during this time will serve you well in future clinical research projects. If you want to learn more about the clinical research profession, please check out our specialized training programs and articles below to learn more about the clinical research job scene. Clinical research coordinators play an important role in ensuring the safety and efficacy of new medical treatments. In order to become a clinical research coordinator, there are a few certification options to consider. The most common is the CCRPS, or Clinical Research Coordinator Professional Society Certification. This certification requires completing a clinical research coordinator training program and passing an exam.
Leads and drives the project team in the planning, execution, and management of all operational aspects of clinical oncology trials. Bachelor's degree and 5+ years of oncology-related work experience. This individual will be responsible for providing coordination and administrative support for a clinical research trial run within the Real-World Evidence Division. Evaluate patients against specific genetic and clinical inclusion and exclusion criteria using various systems.
Remote Senior Clinical Research Regulatory Coordinator
If lead, accountable for the clinical/scientific execution of the protocol. Works extensively with coverage analysis specialists, budget specialists, clinical research coordinators, principal investigators, various stakeholders of UW, the org, and SCCA, to assist with building of calendars in accordance with the protocol... Any site that wants to do research must have a certified clinical trial coordinator and hold investigator meetings.
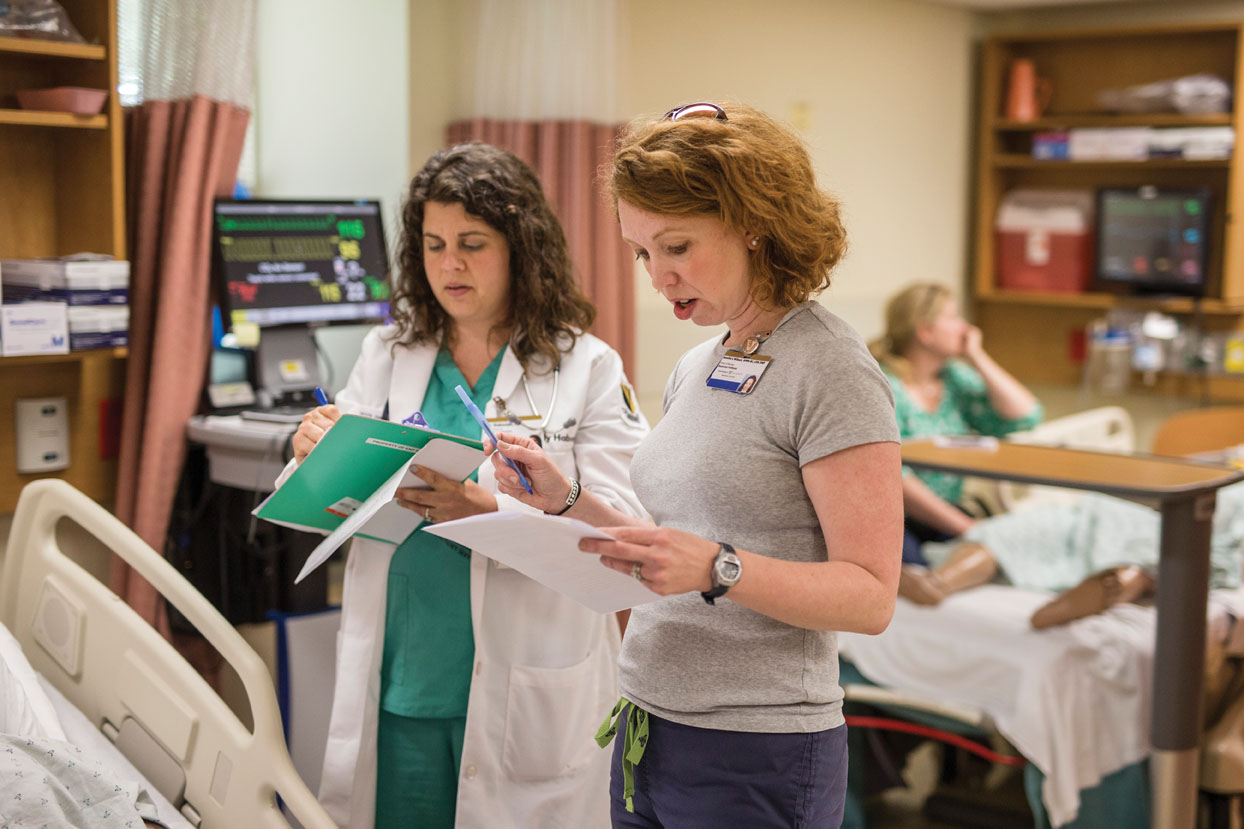
The COVID19 crisis has created a new normal in the workplace. Many companies have required their employees to work remotely as much as possible, and the clinical research field is no different. In this unprecedented time, the FDA has encouraged the use of technology, telehealth, and remote drug deliveries for conducting clinical trials. For many clinical research professionals, the shift to remote work can be challenging and difficult to navigate.
Once they are certified, they will be eligible for clinical research coordinator jobs at hospitals, universities, and other research facilities. Clinical research coordinator salaries vary depending on experience and education, but they typically range from $50,000 to $70,000 per year. With the right education and training, anyone can become a certified clinical research coordinator and start working in this exciting field. A clinical research coordinator is an important member of the research team. The clinical research coordinator is responsible for coordinating all aspects of the clinical trial, from start to finish.
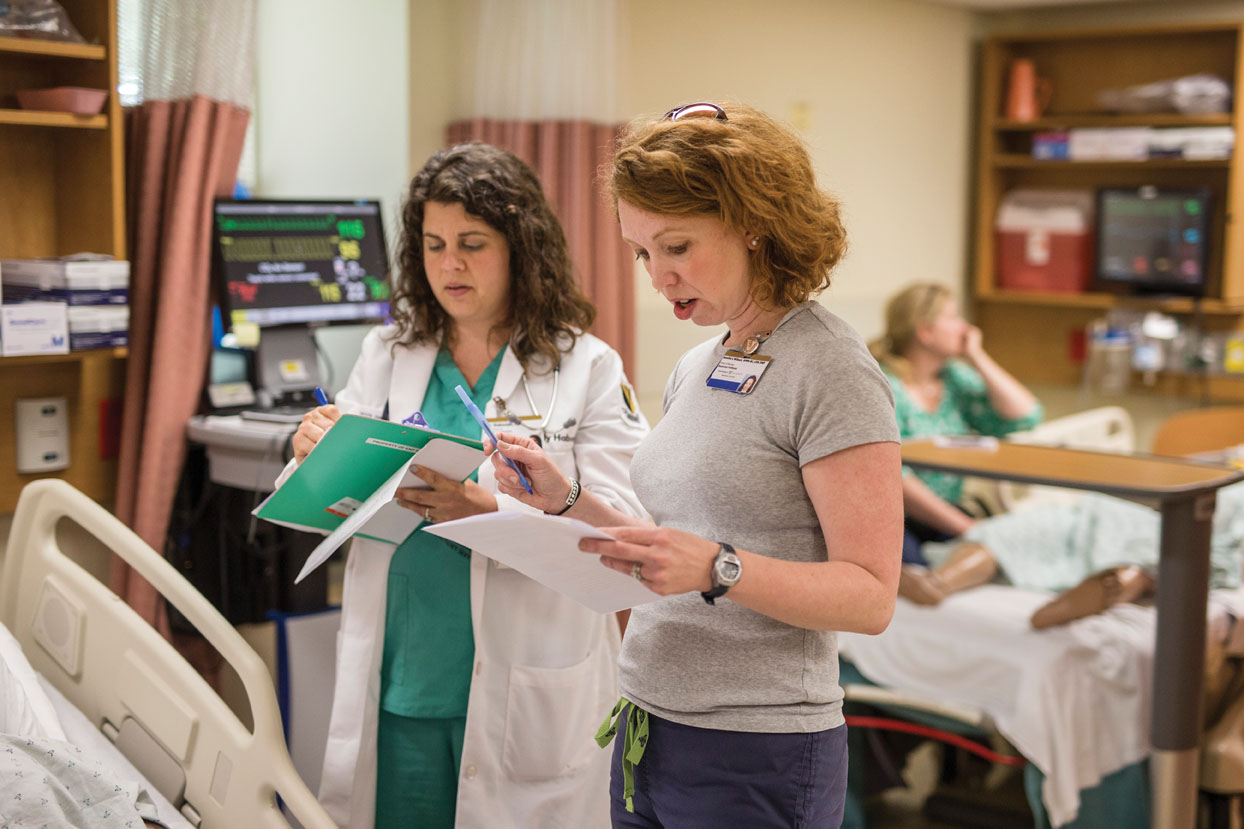
They must also monitor all lab reports and get signatures from the primary investigator. Working in a pandemic is a special circumstance that deserves special considerations from employers. What a lot of people forget is that most companies have created HR guidelines for employees working during a time of crisis. Reviewing these guidelines can help you understand your company’s remote work policies, as well as procedures and expectations. In addition to what is written in these guidelines, it is also important for you to check in with your supervisors and colleagues to confirm what is expected in your department.
If you always talked to a particular colleague before the weekly meeting, try giving them a call before the same virtual meeting. This way, you’ll be able to maintain your old routine and keep up with your office even when you’re working from home. The Clinical Research Manager provides administrative direction for the Pediatric Clinical Trials Office . The Clinical Research Manager will report into the PCTO's Program Manager/Director for clinical research responsibilities. Instructs others in effective practices related to database management. Collaborates with the Principal Investigator in developing modifications to procedures and documentation related to participant engagement in the lab.
Document and communicate study site progress and issues/concerns to the project team. Assist other CRAs with study site issues/concerns/audits with the... The Center is a collaboration between Brown University, Butler Hospital, Lifespan, and Massachusetts General Hospital. Take the next step and explore the options available as a Research Nurse II by joining the company that was voted as one of Fortune 500 s best employers! Our growing Labcorp Drug Development team in our Madison, WI clinic is seeking highly motivated and quality-driven nurses with a passion for clinical care and science who enjoy working in a fast-paced environment. Research, develop and optimize advanced algorithms, actively engage and collaborate with the team and report results to a variety of audiences.
Komentar
Posting Komentar